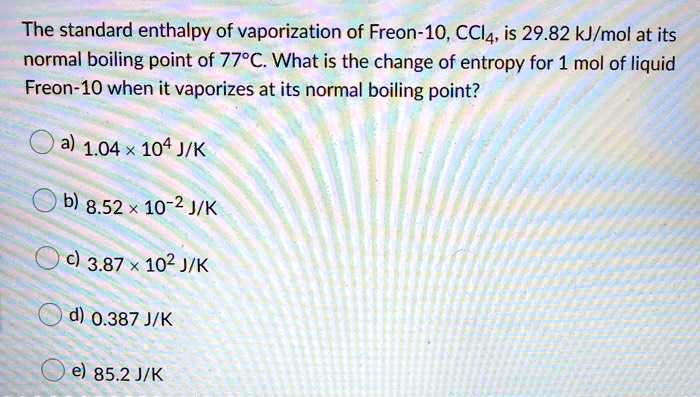Latent heat of vaporization depending on the pressure and R-152a mass... | Download Scientific Diagram

SOLVED:The heat of vaporization of liquid Freon-12, CCl2 F2 is 4.71 kcal / mol. Calculate the energy required to vaporize 39.2 g of this compound. The molecular weight of Freon-12 is 120.9 amu.

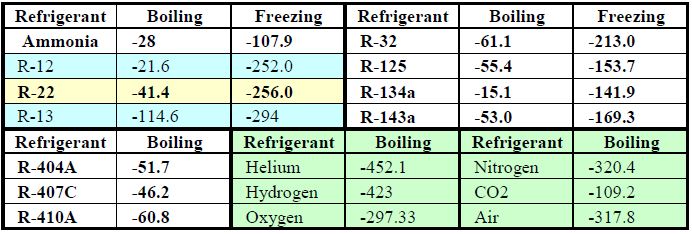
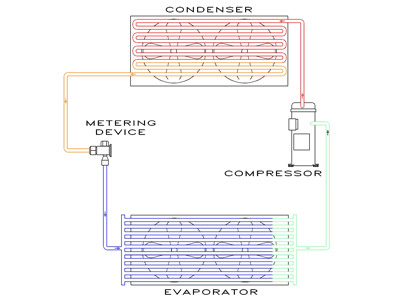
PHYSICAL, CHEMICAL AND THERMODYNAMIC PROPERTIES OF REFRIGERANTS REFRIGERANT: It is any substance that absorb heat through expansion or vaporization and. - ppt download

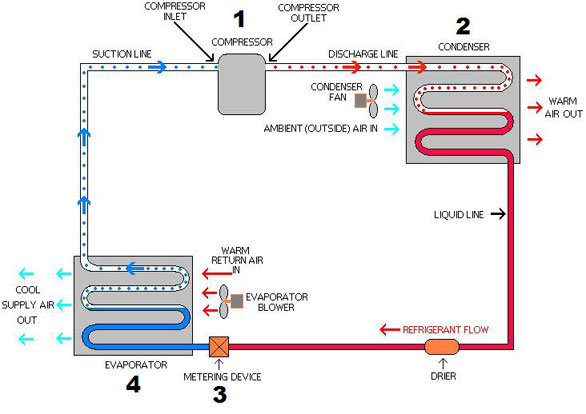
QUIZ 2 A refrigerator uses refrigerant-134a as the working fluid and operates on an ideal vapor-compression refrigeration cycle between 0.18 and 0.9 MPa. - ppt video online download

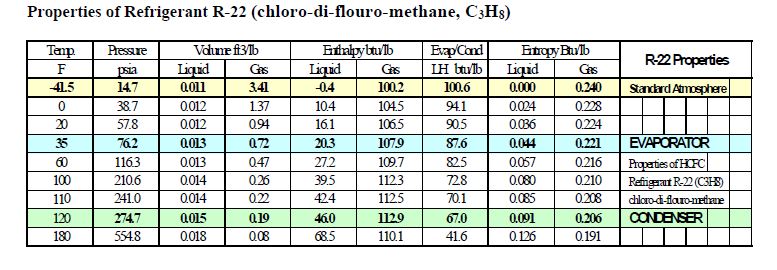
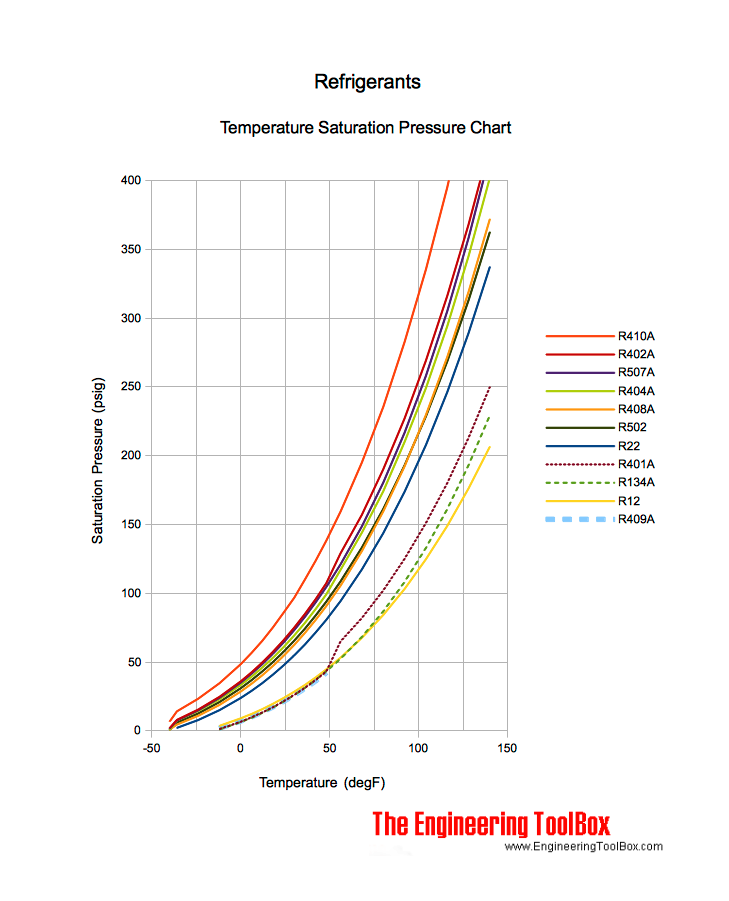
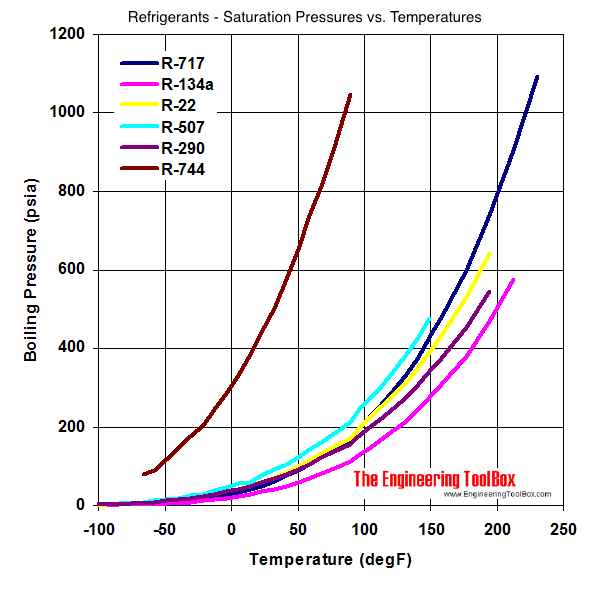
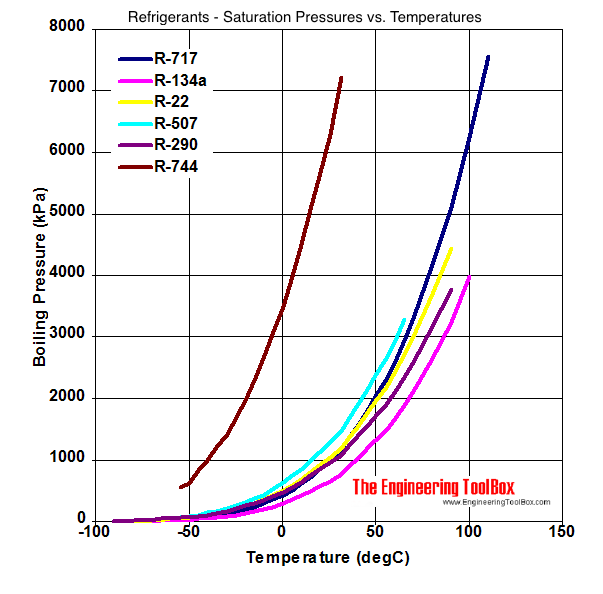
Comparison of evaporation temperature vs. refrigeration duty for R22... | Download Scientific Diagram
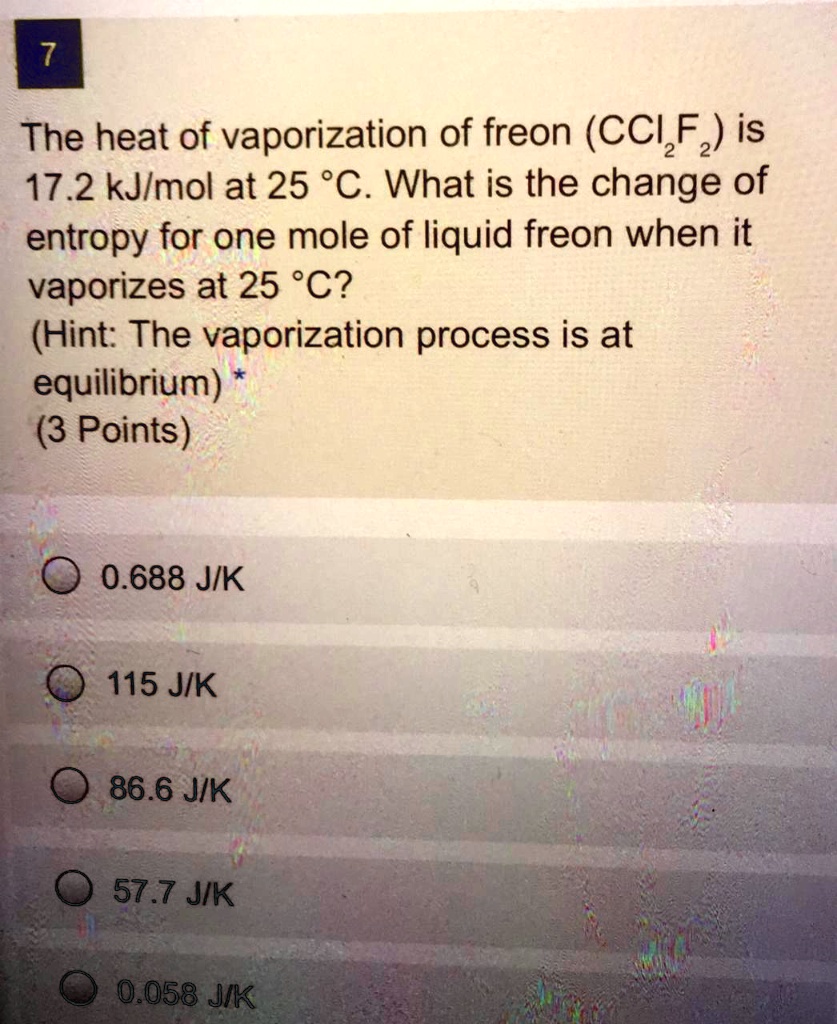
SOLVED: 7 The heat of vaporization of freon (CCI Fz) is 17.2 kJlmol at 25 *C. What is the change of entropy for one mole of liquid freon when it vaporizes at