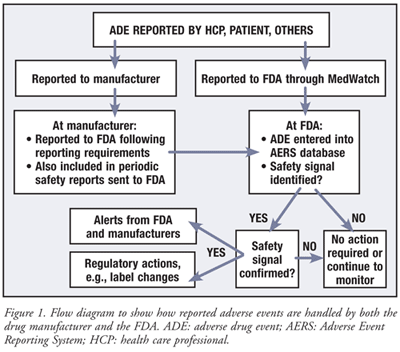
Documenting, Recording, and Reporting of Adverse Events and Unanticipated Problems Introduction Adverse Events Challenges in Onc

National and institutional trends in adverse events over time: a systematic review and meta-analysis of longitudinal retrospective patient record review studies

Identifying adverse drug event information in clinical notes with distributional semantic representations of context - ScienceDirect
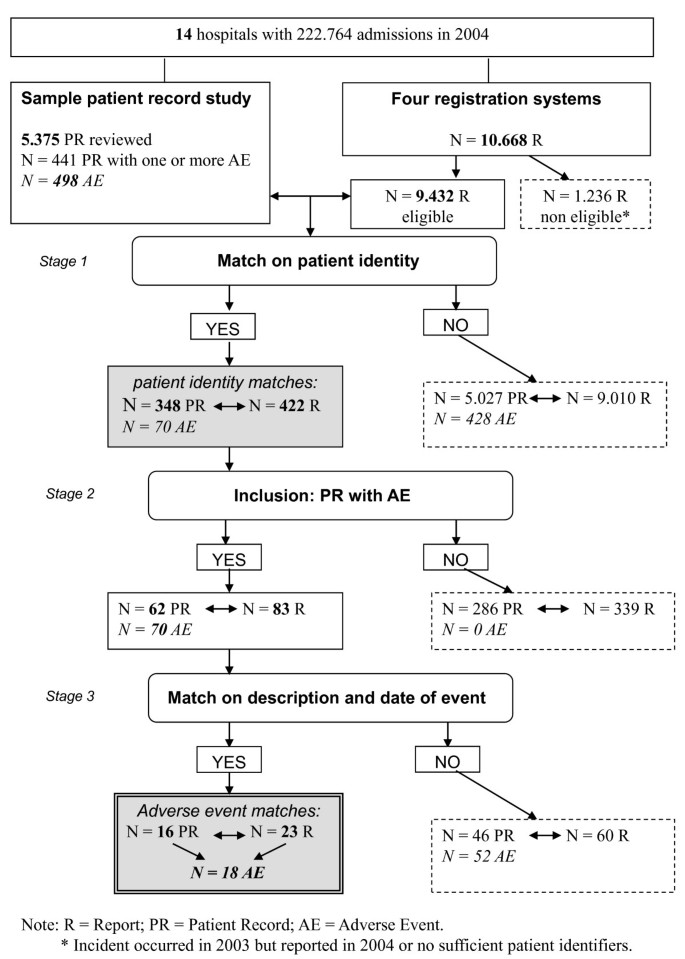
To what extent are adverse events found in patient records reported by patients and healthcare professionals via complaints, claims and incident reports? | BMC Health Services Research | Full Text
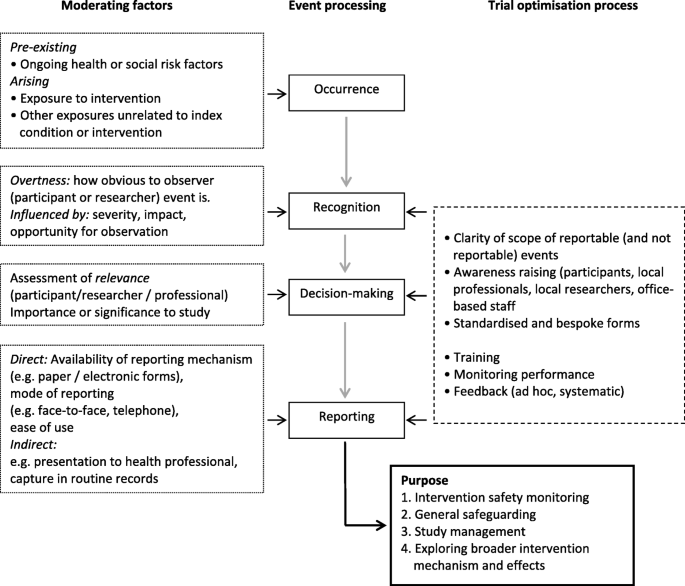
Monitoring adverse social and medical events in public health trials: assessing predictors and interpretation against a proposed model of adverse event reporting | Trials | Full Text

Adverse event (AE) reporting algorithm. Timeframe for adverse event... | Download Scientific Diagram

What are 'adverse events' and why is it necessary to record and report them? Students 4 Best Evidence Tutorials and Fundamentals