John Carlos Baez on Twitter: "Entropy is missing information. But we can measure changes in entropy by doing experiments. So if we assume hydrogen has no entropy at absolute zero, we can

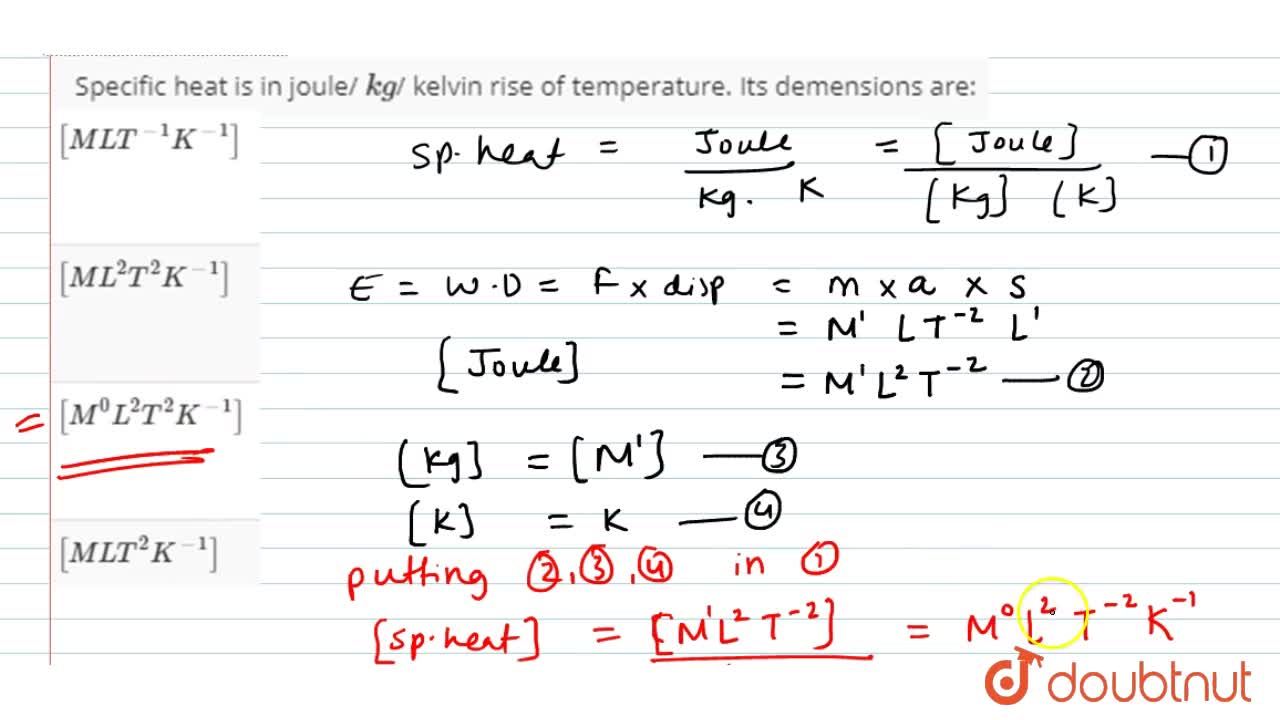
Specific heat of hydrogen at constant pressure, Cp = 29 joule kelvin^-1mol ^-1 (a) Find dimensions of Cp .(b) Unit of length is changed to 50 cm , unit of time is

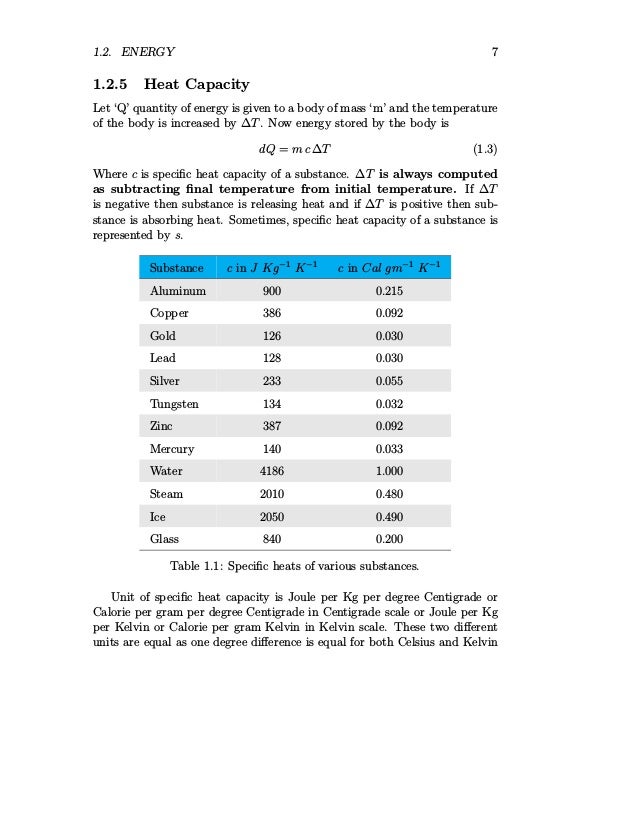
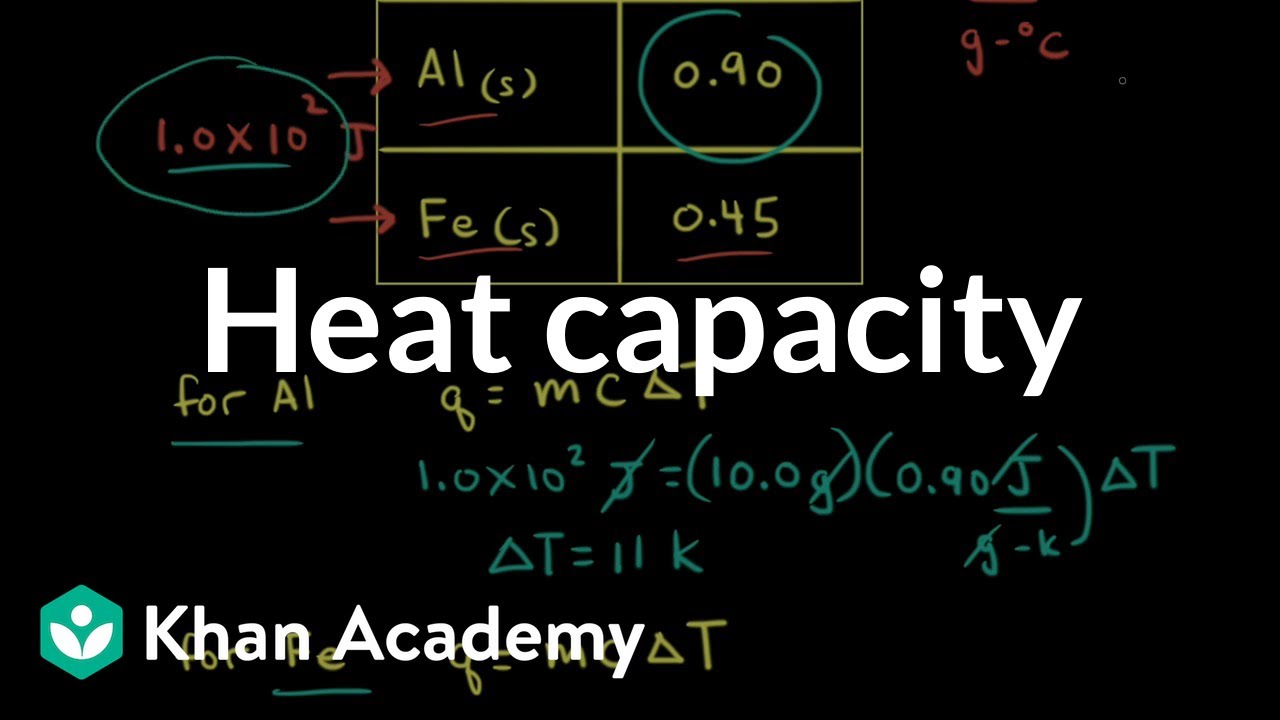
The quantity of energy as heat that must be transferred to raise the temperature of 1 gram (1g) of a substance 1 Kelvin (1K) Specific Heat Definition. - ppt download

SOLVED: A coffee cup calorimeter contains 152.27 g of water at 22.50 %C. A 66.594 g piece of iron is heated to 100.43 'C The piece of iron is added to the
Specific heat of hydrogen at constant pressure is 30 joule per Kelvin per mol. If unit of length changed to 50 cm, unit of time changes to 1/4 sec and unit of
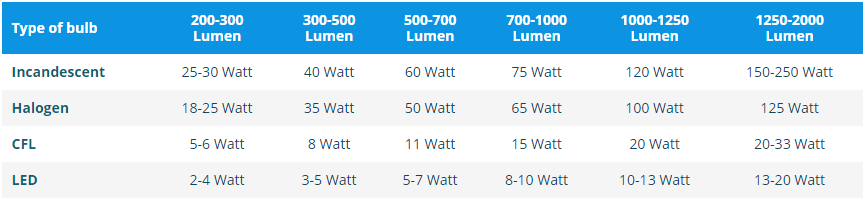
Watt vs. Lumen vs. Kelvin - How to Purchase Lights | SiteLites - Custom Manufacturing & Engineering Inc.