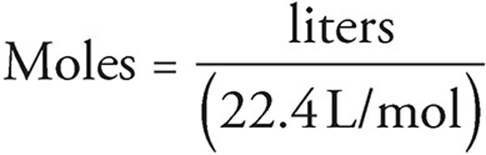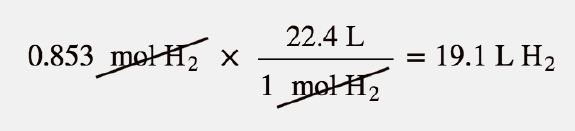Gases & Stoichiometry. Molar Volume 1 mol of gas = 22.4 L molar volume What volume would be occupied by 0.77 moles of helium gas at STP? - ppt download

Gases & Stoichiometry. Molar Volume 1 mol of gas = 22.4 L molar volume What volume would be occupied by 0.77 moles of helium gas at STP? - ppt download

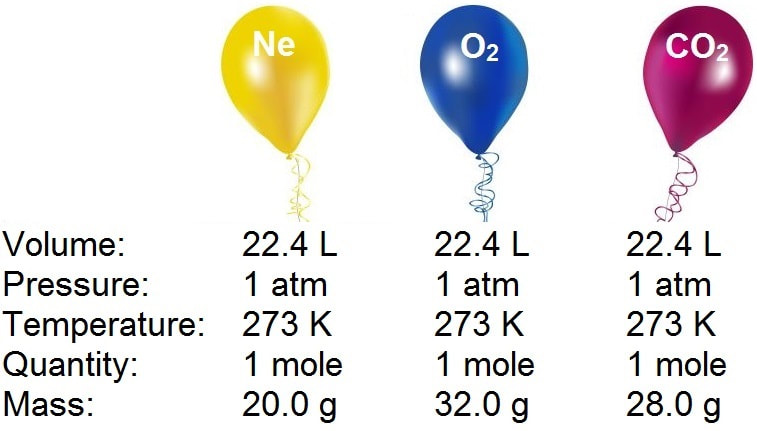
SOLVED:Suppose you have 22.4 L of the following gases at STP: neon, Ne, argon, Ar, and xenon, Xe. a. How many atoms are there in each gas sample? b. What is the

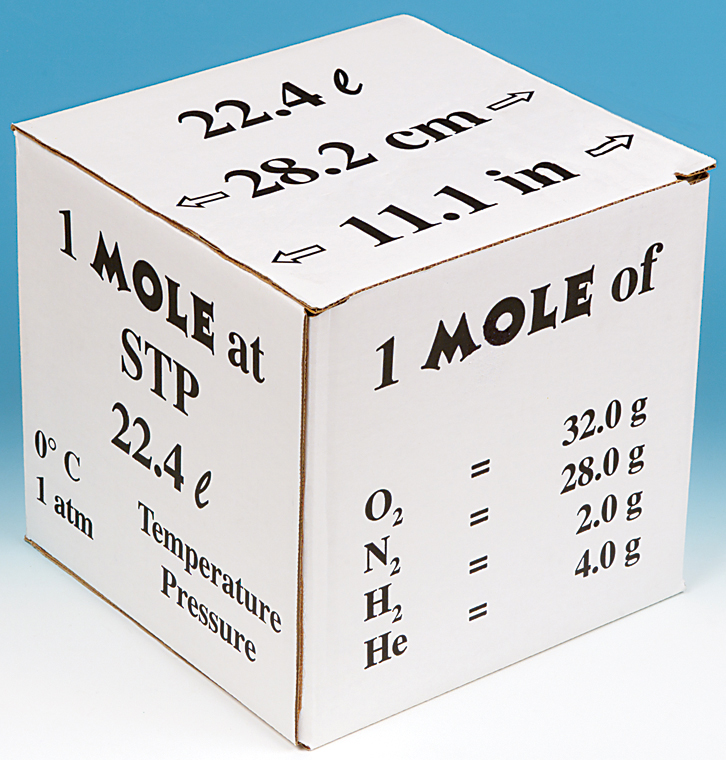
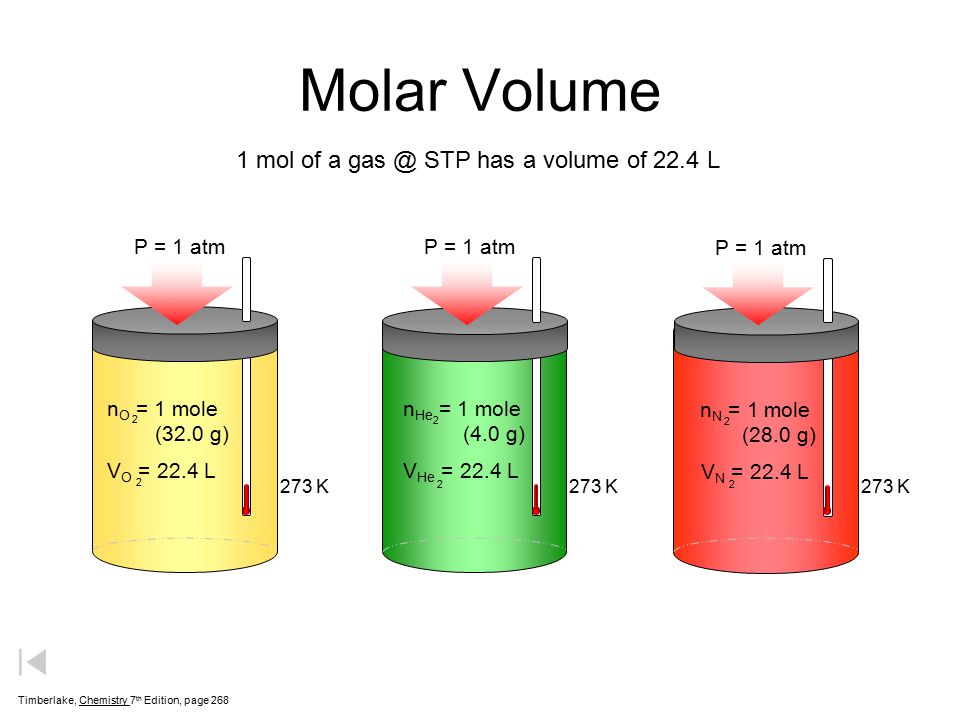
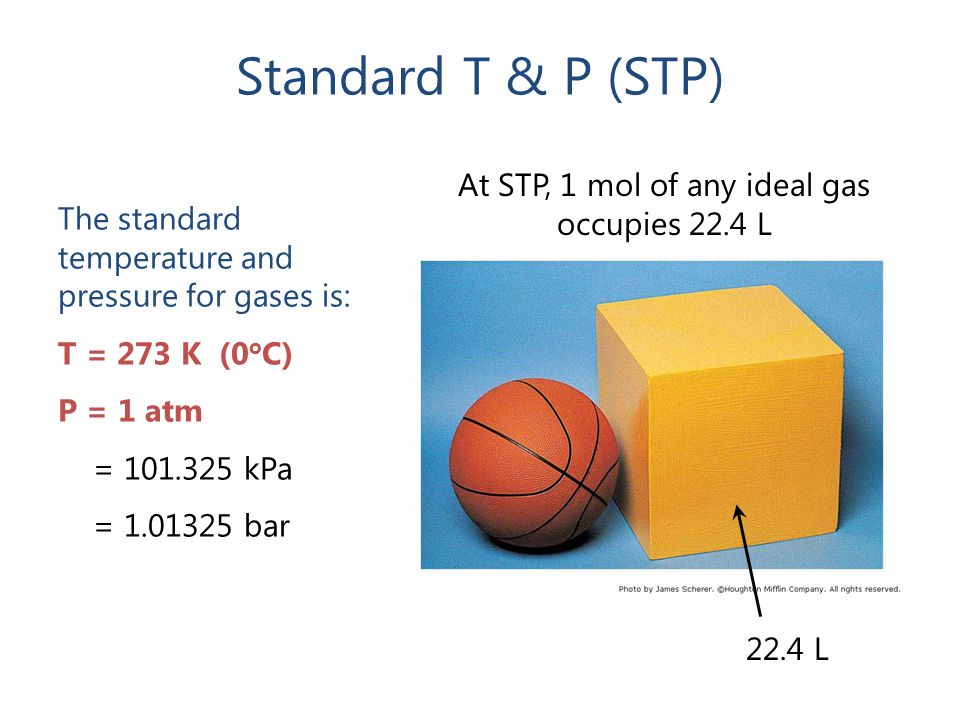
Molar volume is the volume occupied by 1 mol of any (ideal) gas at standard temperature and pressure (STP : 1 atmospheric pressure, 0^oC ). Show that it is 22.4 litres .

By Avogadro's law, V=kn If volume occupied by 1 mole of a gas at STP is 22.4 L, then what will be the value of k if we take 0.5 mole of

One mole of an ideal gas at NTP occupies 22.4 liters (molar volume). What is the ratio of molar ... - YouTube

What volume will 1 mole of a gas occupy at STP? STP = 273K, 1.013x10 5 Pa One mole of any ideal gas occupies a volume of 22.4L at STP. - ppt download