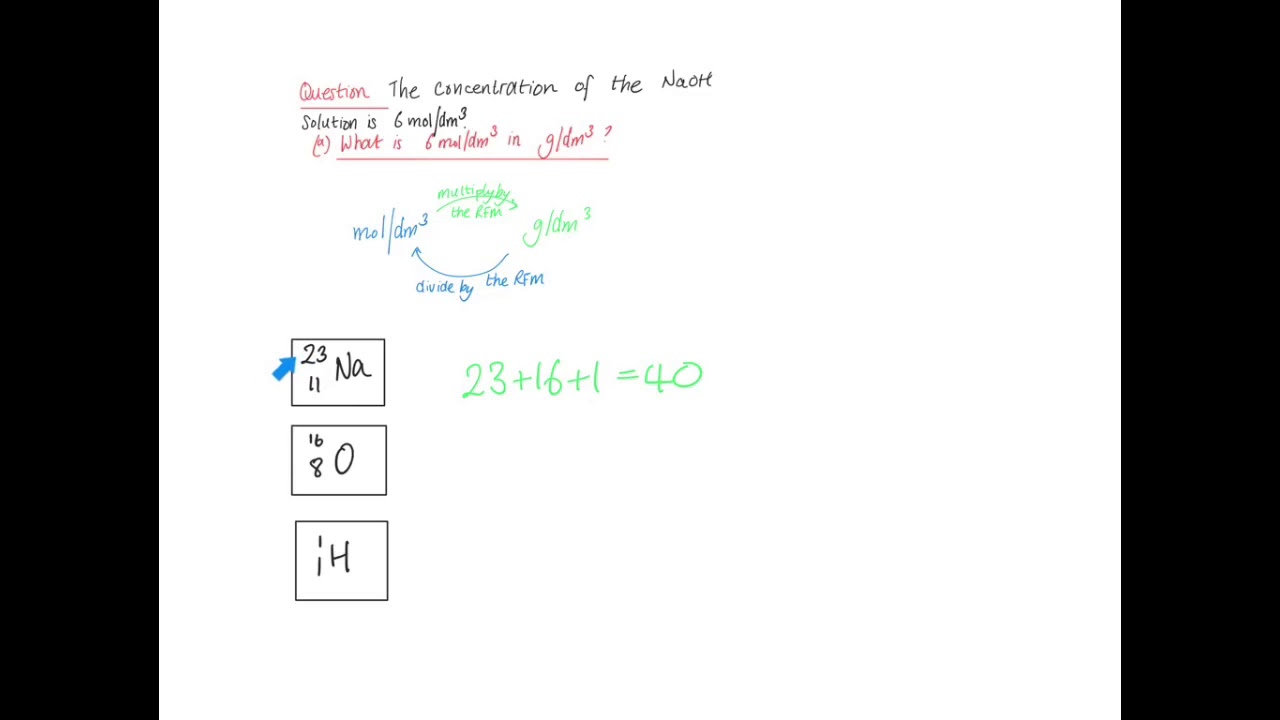
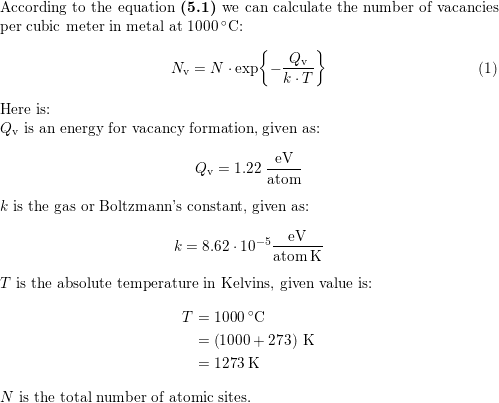The molarity of H2SO4 is 0.8 and its density is 1.06 cm^3. What will be its concentration in terms of molarity and mole fraction? - Quora

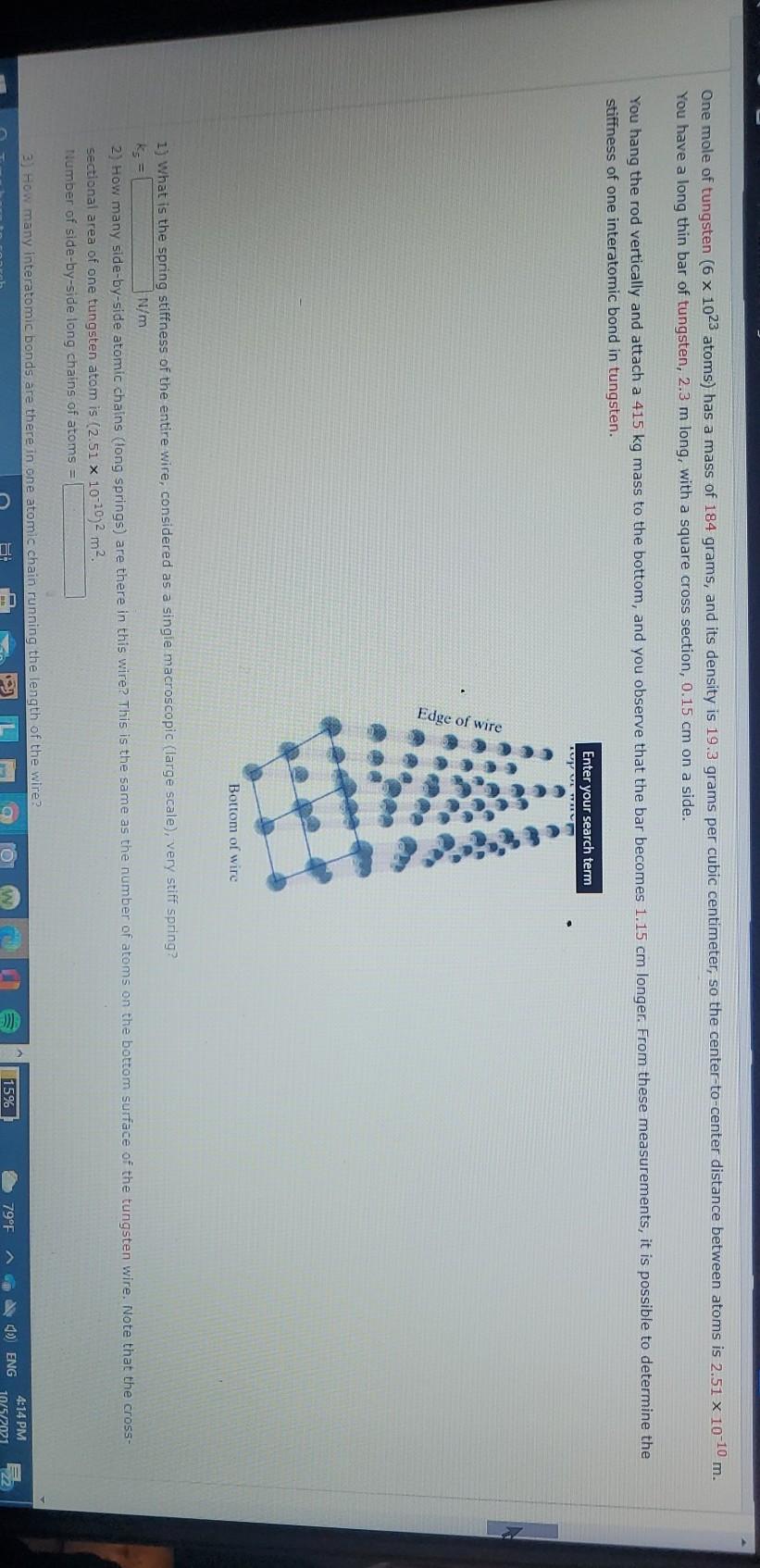
SOLVED:In outer space the density of matter is about one atom per cm^3, mainly hydrogen atoms, and the temperature is about 2.7 K. Calculate the rms speed of these hydrogen atoms, and
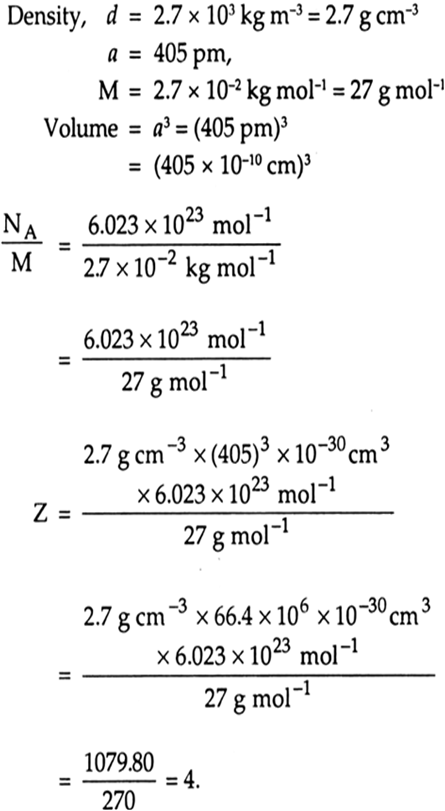
An element with molar mass 2.7 x 10–2 kg mol–1 form a cubic unit cell with edge length 405 pm. If its density is 2.7 x 103 kg m–3. What is the

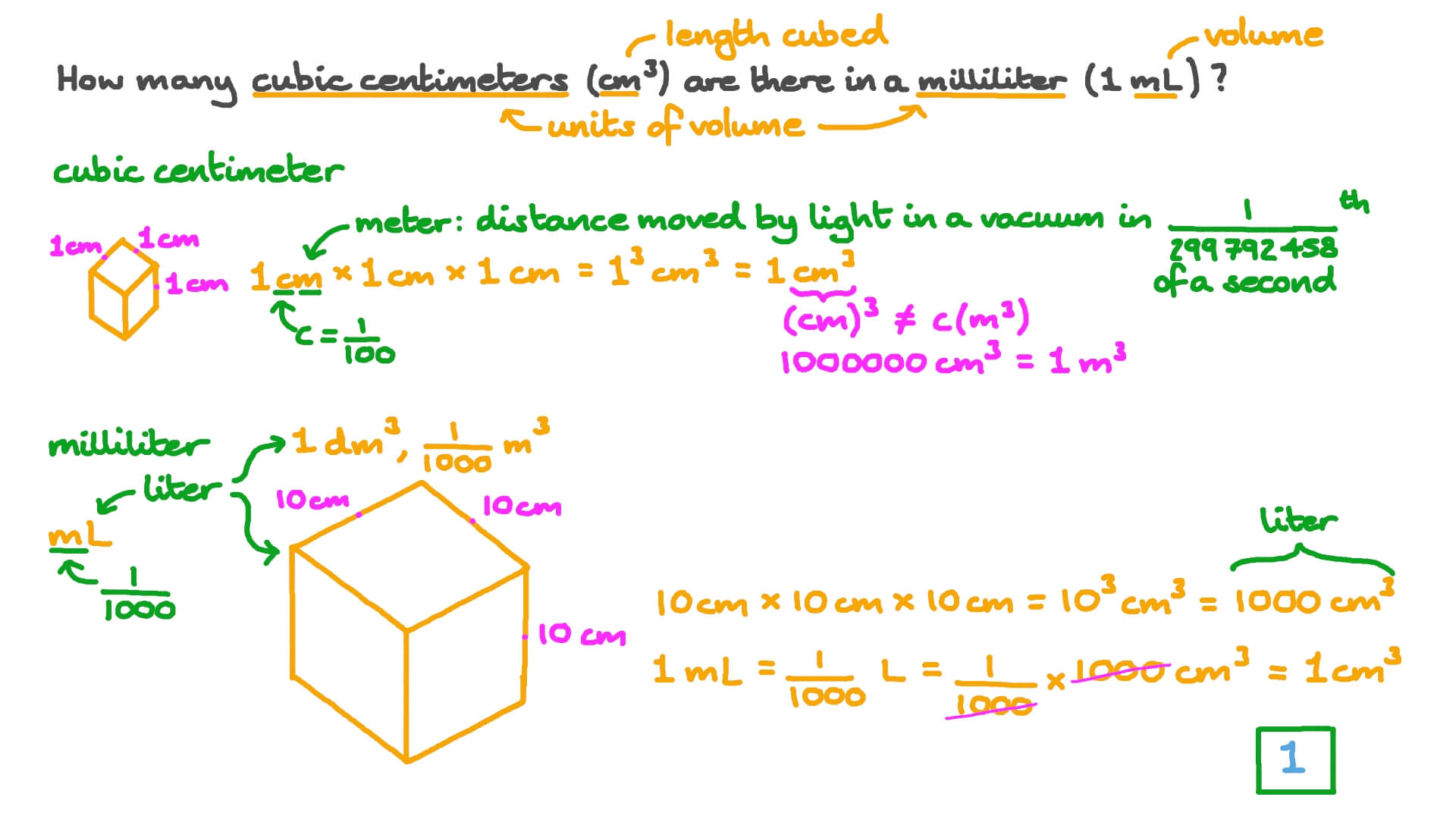
Mole Calculations. Volume, cm 3 Mass, gMolesAtoms use densityuse molar mass use Avogadro's number g cm 3 mol g atoms mol xxx A graduated cylinder holds. - ppt download

Moles and Solutions SPECIFICATIONS Moles and solutions Calculate the amount of substance in moles using solution volume and concentration. - ppt download
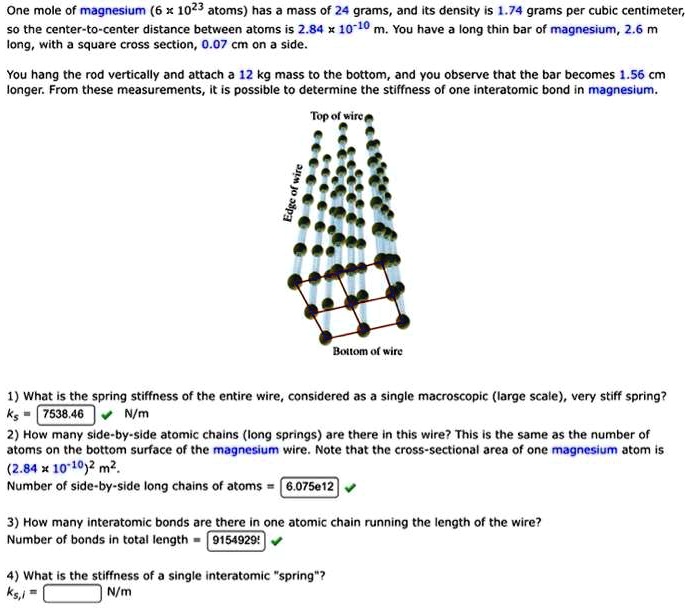
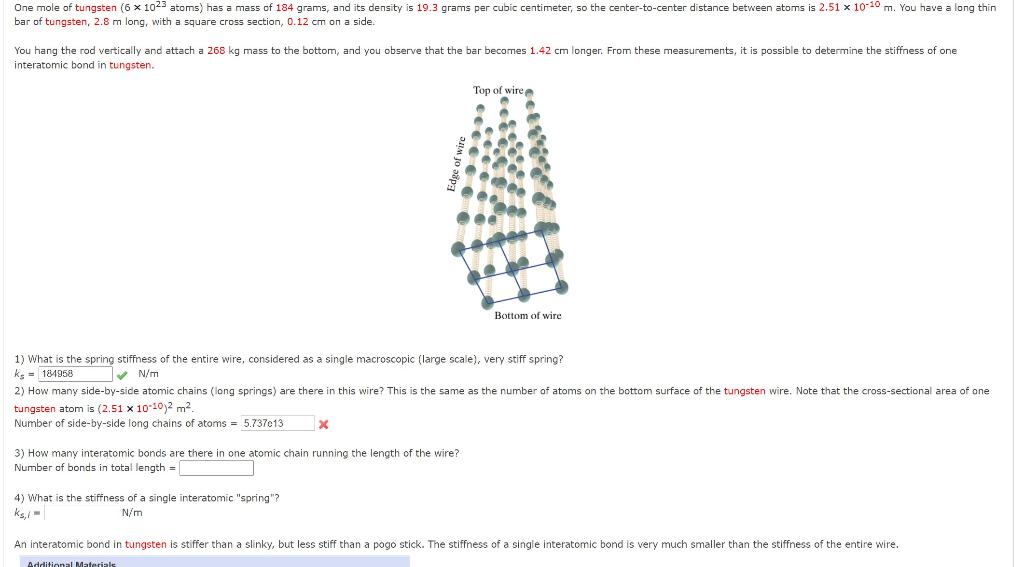
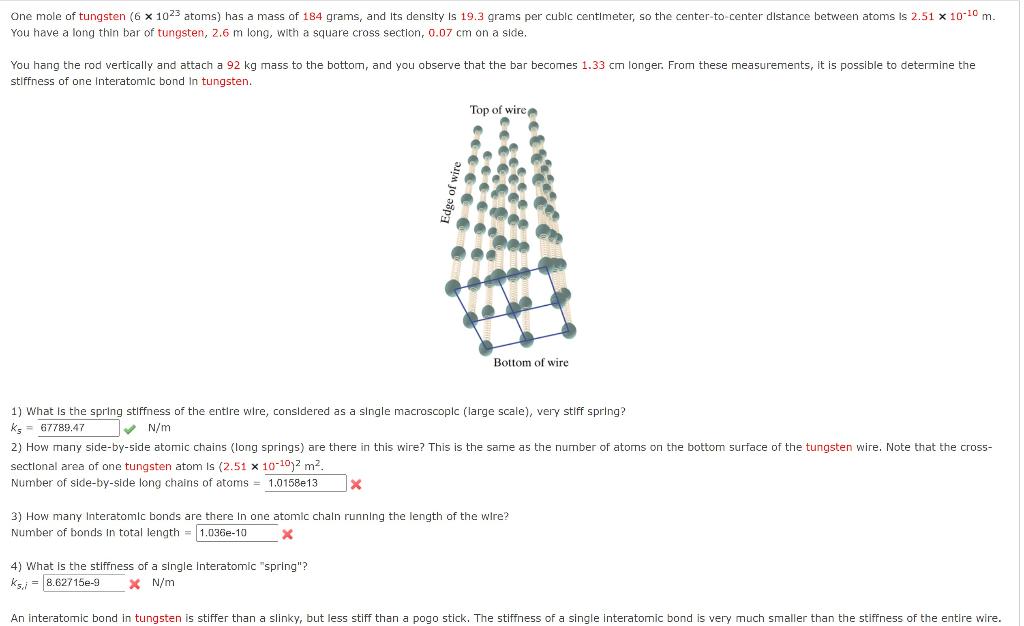
SOLVED: One mole of magnesium (6 1023 atoms) has mass of 24 grams and its density i5 1.74 grams per cubic centimeter; the center-to-center distance between atoms is 2.84 * 10-10 m
A cubic unit cell with an edge length of a cm consists of identical particles. The mass of each particle is m g and the density of the unit cell is (4m)/a^(3)g*cm^(-3).

Sensitivity (in cm 3 ·mol −1 per 0.01 log unit) of the calculated V 1 °... | Download Scientific Diagram